Fuel cell
Fuel cell is the device which converts chemical energy into electrical energy. Hydrogen oxygen fuel cell is one of the examples. It consists of 3 compartments separated by porous carbon electrode. Concentrated KOH or NaOH solution is placed between the electrodes to act as the electrolyte. The hydrogen or oxygen gases are bubbled through the porous electrode into the KOH/NaOH solution.
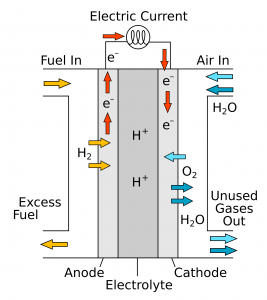
Working of Fuel Cell:
Concentrated KOH or NaOH solution is placed between the electrodes to act as the electrolyte. The hydrogen or oxygen gases are bubbled through the porous electrode into the KOH/NaOH solution.
Reactions:-
At Anode: – 2H2 + 4OH- –> 4H2O + 4e-
At Cathode: – O2 + 2H2O + 4e- –> 4OH-
Overall reaction: 2H2(g)+O2(g)2H2O
Types of Fuel Cells:
Fuel Cells are Electro chemical Cells and have various types. They are sated below with brief .
1. Polymer Electrolyte Membrane Fuel cell
2. Phosphoric Acid Fuel Cells
3. Solid Acid Fuel Cells
4. Molten Carbonate Fuel Cell
Polymer Electrolyte Membrane Fuel cell: It is also called as Proton exchange membrane fuel cells and used to transfer applications, portable fuel cell applications, and stationary fuel cell applications. They are characterized by features such as polymer electrolyte membrane and pressure that range from 50° to 100° Celsius.
Phosphoric Acid Fuel Cells: In this fuel cell, the liquid phosphoric acid is used as an electrolyte. The electrode is composed of carbon paper and It involves the following electrode reactions.
Anode: 2H2(g) → 4H+ + 4e‾
Cathode: O2(g) + 4H+ + 4e‾ → 2H2O
Overall: 2 H2 + O2 → 2H2O
These are used in bus and stationary power generators. One of the disadvantages of phosphoric acid fuel cells is use of an acidic electrolyte. Because when components are exposed to phosphoric acid, it leads to corrosion.
Solid Acid Fuel Cells: The electrolyte of the solid fuel cell is form of solid acid material. It possesses a molecular structure similar to that of salts at low temperature
Reactions for solid acid fuel cells
Anode: H2 → 2H+ + 2e−
Cathode: ½O2 + 2H+ + 2e− → H2O
Overall: H2 + ½O2 → H2O
These are especially used for remote places in military applications.
Molten Carbonate Fuel Cell: Molten carbonate fuel cells are operate at a high temperature of six hundred degree Celsius. They are presently designed for military, natural gas, biogas, industries, and coal-based power plants and the electrode of a molten carbonate fuel cell is made up of molten carbonate salt mixture.
Applications of fuel cells:
- Fuel cells have many advantages over conventional cells as they produce very less amount of harmful greenhouse gases. The by-product of fuel cells is only heat and water.
- Fuel cells are portable so it can be taken anywhere. This helps in military applications such as for remote places and they can also be used as torches, electronic gadgets like camera, laptops etc.
- Now automobile industries are planning to launch the vehicles which are run by fuel cells due to less cost.




